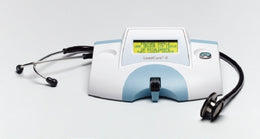
Abbott Rapid Dx North America LLC Respiratory Test Kit ID NOW™ Molecular Diagnostic COVID-19 2.0 Nasal Swab / Nasopharyngeal Swab Sample 24 Tests - KT/1 - 192000-KT
Our Policies
Privacy policy
Payment policy
Shipping policy
Regular price
$2,314.99
-Liquid error (snippets/product-price line 34): Computation results in '-Infinity'%
Regular price
$2,314.99
Certain items may require either a prescription, medical license, or a business facility license to process. If your product falls in that category, we will reach out to you to obtain proper documentation.
If you already have a prescription, you may email us at support@healthcareorigin.com along with the order number.
- Description
- ID NOW COVID-19 2.0 Assay is for use under an Emergency use Authorization Only: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-molecular-diagnostic-tests-sars-cov-2
- To utilize the ID NOW COVID-19 2.0 assay, a software upgrade to version 7.0.0.20 is required for all instruments; the software upgrade is backward compatible to support use of ID NOW COVID-19, Influenza A & B 2, Strep A 2, and RSV inventory
- Product ships with minimum 30 days dating
- ID NOW COVID-19 2.0 assay performed on the ID NOW Instrument is a rapid molecular in vitro diagnostic test utilizing an isothermal nucleic acid amplification technology (NAAT) intended for the qualitative detection of nucleic acid from SARS-CoV-2 in direct anterior nasal (nasal) or nasopharyngeal swab specimens from individuals who are suspected of COVID-19 by their healthcare provider within the first seven days of the onset of symptoms
- Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform high, moderate or waived complexity tests
- The ID NOW COVID-19 2.0 assay is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation
- Positive results are indicative of the presence of SARS-CoV-2 RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status
- Positive results do not rule out bacterial infection or co-infection with other viruses
- Testing facilities within the United States and its territories are required to report all results to the appropriate public health authorities
- Negative results should be treated as presumptive and, if inconsistent with clinical signs and symptoms or necessary for patient management, should be confirmed with a different authorized or cleared molecular test in a CLIA-certified laboratory that meets requirements to perform high or moderate complexity tests
- Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions; negative results must be combined with clinical observations, patient history, and/or epidemiological information
- Materials required but not provided: ID NOW Instrument, Nasopharyngeal Swabs
- To utilize the ID NOW COVID-19 2.0 assay, a software upgrade to version 7.0.0.20 is required for all instruments; the software upgrade is backward compatible to support use of ID NOW COVID-19, Influenza A & B 2, Strep A 2, and RSV inventory
- ID NOW COVID-19 2.0 is intended for use by trained operators who are proficient in performing tests using the ID NOW Instrument
- Positive results as early as 6 minutes, negative results in 12 minutes
TEST KIT, COVID-19 ID NOW 2.0 ASSAY
| Item Id | 1213540 |
| MF Id# | 192000 |
| Brand | ID NOW™ |
| Manufacturer | Abbott Rapid Dx North America LLC |
| Country of Origin | Unknown |
| Application | Respiratory Test Kit |
| CLIA Classified | CLIA Waived |
| Contents 1 | Test Bases, Sample Receivers, Transfer Cartridges, Patient Swabs, Positive Control Swab, Negative Control Swab, Package Insert, Quick Reference Instructions |
| For Use With | For ID NOW™ Instrument |
| Is_Active_Vendor | Y |
| Is_DSCSA | N |
| Is_Discontinued | N |
| Is_Medical_Device | N |
| Lot_Tracking_Flag | Y |
| Number of Tests | 24 Tests |
| On_Allocation | N |
| Product Dating | McKesson Acceptable Dating: we will ship >= 30 days |
| Purchase Program Type | Standard Purchase |
| Reading Type | Machine Read |
| Sample Type | Nasal Swab / Nasopharyngeal Swab Sample |
| Specialty | Molecular |
| Supplier_ID | 64711003 |
| Technology | Isothermal Nucleic Acid Amplification Technology (NAAT) |
| Test Format | Cartridge Format |
| Test Name | COVID-19 2.0 |
| Test Type | Molecular Diagnostic |
| Time to Results | 12 Minute Results |
| UNSPSC Code | 41116144 |